See also |
|||
The most effective method to control undesirable effects of metal corrosion is Cathodic protection. This technique, used worldwide since the 1930’s was originally introduced in the early nineteenth century to meet the needs of the British Navy and also used, with excellent results, for the protection of underground structures.
The first property to consider is their electrical potential. All metals generate a negative voltage (as compared to a reference electrode) when immersed in water. The lower – the more negative - the voltage, the more active the metal is considered to be, for example: The best anodes are made from an "activated alloy" in which catalysts have been added to ensure that the anode gives 100% of itself in the purpose of protecting your boat from corrosive action. What is Galvanic Corrosion? If you've noticed corrosion on the metal parts of your boat located below the waterline, you are the victim of "galvanic corrosion". The scientific term "galvanic" corrosion describes the type of corrosion that anodes are intended to absorb. This corrosion is normally caused by different metals being near each other in salt water. Galvanic corrosion, an electromechanical action, causes metal parts to decompose. This destructive process is caused by electrolysis, an electric current set up between the metal parts of your boat, with salt water as the electrolyte. The effect is like a flashlight battery -- an electrical current is created and continues until one of the metals is eaten up -- the battery goes "dead". What is Electrolytic Corrosion? If you see an accelerated corrosion on the metal parts of your boat located below the waterline, you are the victim of "electrolytic corrosion". The scientific term "electrolytic" corrosion describes an accelerated type of corrosion that occurs when an electric current is added to the water surrounding your boat (usually at a dock). This corrosion is typically caused by faulty wiring that permits an electric current to enter the water. This corrosion, combined with galvanic corrosion, is also an electromechanical action which causes metal parts to decompose, but at a very accelerated level. This destructive process is also caused by electrolysis, which is an electric current set up between the metal parts of your boat, with salt water as the electrolyte, but it can be much more damaging in a very short time. The protection of the underwater metal parts on your boat or motor from corrosive electrolytic action is a very real and necessary concern. The use of an activated alloy such as an “anode” will help. Anodes will distract the corrosion action to itself and away from expensive metal parts. Fortunately anodes are relatively inexpensive and are readily available. Factors such as warm water temperatures, polluted water and stray current corrosion can cause your anodes to waste away at an accelerated rate so it is wise to check them on a regular basis.
An important thing to remember is that you must complete a "circuit" for the anode to be effective, which means that the zinc must be in direct contact with the metal components of your boat located below the waterline. This is the reason that a hanging grouper-style of anode comes with a wire and metal clip that is intended for attachment to a "ground" within your boat. When attaching a zinc anode to a prop or shaft, please be certain that the point of surface attachment is clean and "bright" so a good contact is made, completing the circuit. Also, never paint over a zinc with bottom paint, which will only isolate and protect the anode from its intended purpose. Products

UNIVERSAL ANODE
43.610.00
Special Price: 9.95 €
(Normal Price: 20.46 €)


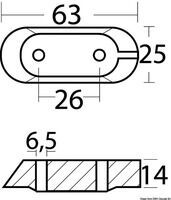
PLATE ANODE, 80 x 55 mm
43.600.05
Special Price: 8.95 €
(Normal Price: 17.50 €)
|
| « Continue shopping |